Abstract
Introduction: Concizumab is an anti-tissue factor pathway inhibitor (TFPI) monoclonal antibody in phase 3 clinical development as a once-daily, subcutaneous prophylaxis across all hemophilia subtypes. The phase 2 trials explorer4 (NCT03196284) and explorer5 (NCT03196297) investigated the efficacy and safety of daily subcutaneous concizumab prophylaxis in patients with hemophilia A/B with inhibitors and in patients with severe hemophilia A without inhibitors, respectively. Minor surgeries and diagnostic procedures were permitted during both trials, allowing for the assessment of conducting such procedures during concizumab prophylaxis.
Objective: The aim of this analysis was to describe surgeries and diagnostic procedures performed while on concizumab prophylaxis during the main and extension parts of the concizumab phase 2 trials.
Methods: Both the explorer4 and explorer5 trials consisted of a main and an extension part, with patients receiving concizumab at an initial maintenance dose of 0.15 mg/kg (with an additional loading dose of 0.5 mg/kg as a first dose in explorer4) in both trials, with the option to dose escalate to 0.20 and 0.25 mg/kg in case of ≥3 spontaneous treated bleeds within the previous 12 weeks. Concomitant treatment with systemic anti-fibrinolytics was not allowed in either trial, while local/topical use was permitted. Minor surgery, defined as an invasive operative procedure in which the skin, mucous membranes, or superficial connective tissue are manipulated, such as skin biopsies, implanting of central venous access devices, or simple dental procedures, were allowed at the investigator's discretion during both trials. Major surgery was not permitted and thus constituted a protocol deviation. Only patients who had been receiving concizumab as part of explorer4 or explorer5 up until, during and immediately after surgery were included in this analysis.
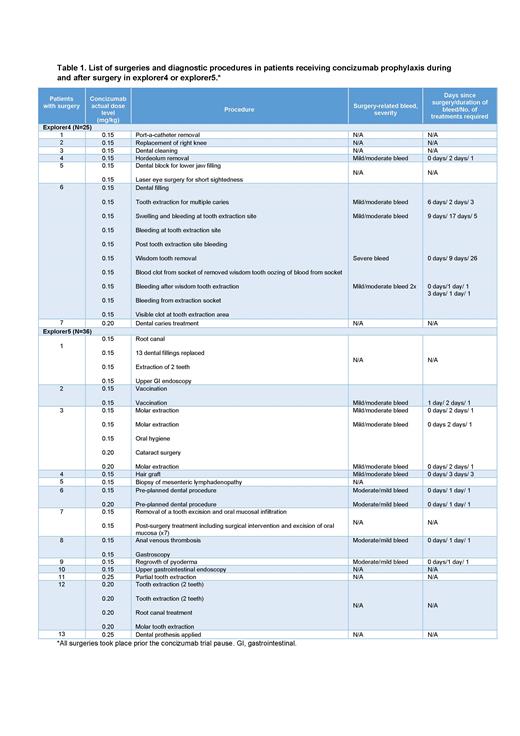
Results: Seven of the 25 patients treated with concizumab in explorer4 had a total of 17 surgeries while treated with concizumab during the trial. The patients who underwent surgery were aged between 24 and 45 years old. Five of these patients underwent a single procedure, 1 patient 2 procedures, and 1 patient underwent a total of 10 procedures while in the trial. At the time of surgery, with the exception of 1 patient who was receiving concizumab at 0.20 mg/kg, all patients were on 0.15 mg/kg. Additional perioperative hemostatic treatment was permitted and was given at the investigator's discretion. The majority of procedures involved dental surgery, although a venous catheter removal, hordeolum removal, and laser eye surgery were also performed (Table 1). Out of a total of 6 recorded surgery-related bleeding episodes in patients receiving concizumab in explorer4, 1 was classified as severe, while the remaining 5 as mild or moderate.
In explorer5, 13 of the 36 patients treated with concizumab (26-65 years old) underwent a total of 33 surgeries while receiving concizumab prophylaxis. Seven of these patients had more than 1 surgery, ranging between 2 and 8 per person. The majority of patients (8/13) were receiving concizumab at 0.15 mg/kg, 1 patient at 0.20 mg/kg and 2 patients at 0.25 mg/kg at the time of surgery, and 2 patients were on 0.15 mg/kg concizumab for their first surgeries and switched to 0.20 mg/kg for later surgeries. Additional hemostatic treatment was given at the investigator's discretion. Dental procedures constituted most surgeries, and other procedures included vaccinations and cataract surgery, diagnostic procedures such as biopsy, endoscopy and gastroscopy, as well as a hair graft (Table 1). A total of 9 surgery-related bleeds were recorded during explorer5, all of which were classified as mild or moderate.
Conclusion: Across both concizumab phase 2 trials, between 28-36% of all patients (7/25 in explorer4 and 13/36 in explorer5) receiving daily concizumab prophylaxis underwent 1 or more surgeries during the overall trial periods of 18 and 22 months, respectively. A wide range of surgeries, including invasive diagnostic procedures, were conducted while the number of surgery-related bleeds was low and classified as mild or moderate, with 1 exception. Prophylactic treatment with concizumab in patients with hemophilia is currently being investigated further in ongoing phase 3 trials.
Wheeler: Novo Nordisk A/S: Consultancy; Bayer: Consultancy; BioMarin: Consultancy; HEMA Biologics: Consultancy; Spark: Consultancy; Takeda: Consultancy; UniQure: Consultancy. Benson: Novo Nordisk A/S: Consultancy, Speakers Bureau; Takeda: Speakers Bureau; BMS: Speakers Bureau; Sobi: Speakers Bureau. Eichler: Pfizer: Research Funding; Novo Nordisk: Consultancy, Research Funding; BioMarin: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; Takeda: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; CSL Behring: Consultancy, Honoraria; Biotest: Consultancy, Honoraria. Marie Tønder: Novo Nordisk Health Care AG: Current Employment. Cepo: Novo Nordisk A/S: Current Employment. Jimenez Yuste: Novo Nordisk A/S: Consultancy, Research Funding; Bayer: Consultancy; Takeda: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Grifols: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Sobi: Consultancy, Research Funding; CSL Behring: Consultancy; BioMarin: Consultancy. Kavakli: Novo Nordisk A/S: Consultancy, Other: Clinical Trial Support; Takeda: Consultancy, Other: Clinical Trial Support; Roche: Consultancy, Other: Clinical Trial Support. Wong: Astellas Pharma, INc.: Research Funding. Matsushita: Baxalta/Shire/Takeda: Consultancy, Honoraria; Bayer: Consultancy; Novo Nordisk A/S: Consultancy, Honoraria, Other: educational and investigational support ; Chugai: Consultancy, Honoraria, Other: educational and investigational support ; Pfizer: Consultancy; Bioverative/Sanofi: Honoraria; CSL: Honoraria; JB: Honoraria; KMB: Honoraria; Kirin: Honoraria; Nichiyaku: Honoraria; Octapharm: Honoraria; Sysmex: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal